Industry Approaches to Science on Newer Products
This page was last edited on at
Key Points
- In 2012, the Institute of Medicine (IOM) published guidance for assessing tobacco and nicotine products proposed by manufacturers as less harmful alternatives to conventional cigarettes.
- Transnational tobacco companies have developed similar multi-stage approaches to scientifically assess these newer products.
- There is evidence to suggest the industry’s approach does not guarantee good quality research or prevent the industry from using strategies to influence science.
- The Framework Convention on Tobacco Control calls for regulatory decisions on tobacco products and the scientific assessment of tobacco products to be made independent of the tobacco industry
Background
In 2003 and 2004, the World Health Organization’s Scientific Advisory Committee on Tobacco Product Regulation (SACTob) and Study Group on Tobacco Product Regulation (TobReg) issued principles and guidance on the type of evidence required to scientifically assess newer tobacco and nicotine products that were purportedly less harmful than cigarettes.12 Both emphasised the need for a range of study types and independent verification of industry studies. In 2012, the US Institute of Medicine (IOM, now The National Academy of Medicine), an independent, evidence-based advisor on scientific, medical and health-related matters,3 outlined the types of studies and appropriate designs, which would be necessary to demonstrate whether newer nicotine and tobacco products could reduce the harms associated with smoking. The IOM grouped the evidence required into three categories: health effects, addictive potential, and perceptions of the newer product.4
In 2015, the Tobacco Product Assessment Consortium (TobPRAC – an independent body funded by the US National Cancer Institute)5 reviewed the WHO’s and IOM’s recommendations for scientific evaluation of purportedly less harmful tobacco products.6 In 2011, the consortium had developed a four-staged framework for scientifically evaluating newer tobacco and nicotine products, particularly those claimed by manufacturers to be less harmful.7 The four stages proposed were: pre-market evaluation, pre-claims evaluation, post-market activities, and monitoring and re-evaluation (see image 1).67
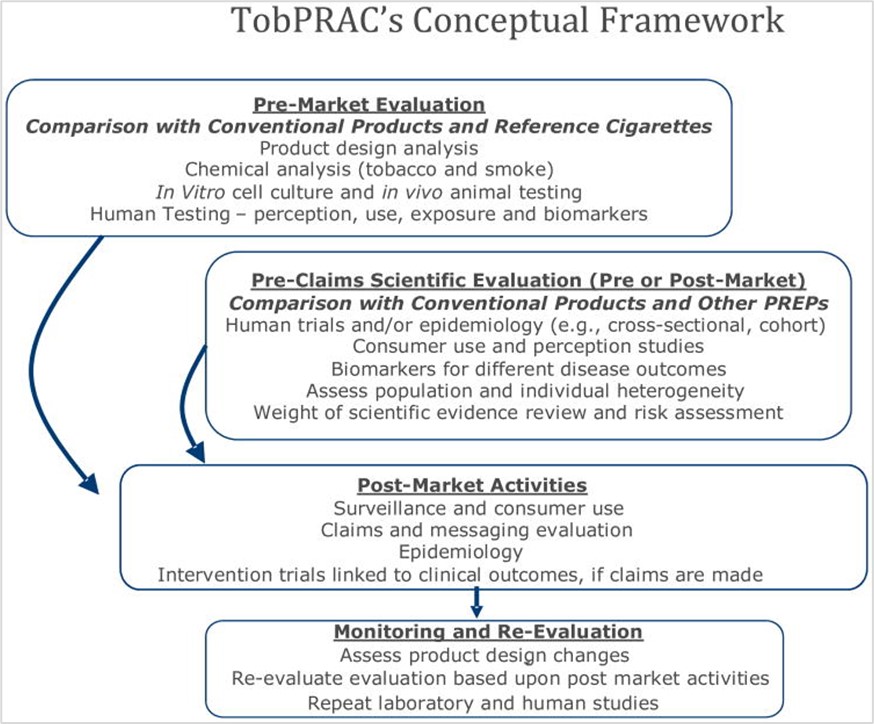
Image 1. Overview of the conceptual framework proposed by the Tobacco Product Assessment Consortium (TobPRAC) to assess newer products.(Source: Shields et al/TobPRAC, 2011, p. 47; Berman, et al, 2015)67
Designed to inform tobacco product regulators worldwide, the framework was not based on any particular regulatory structure and, according to the TobPRAC, is therefore applicable to any jurisdiction. In line with Article 5.3 of the World Health Organization Framework Convention on Tobacco Control (WHO FCTC), TobPRAC noted that regulatory decisions on the necessary criteria for scientifically assessing tobacco products should be made independent of the tobacco industry. As the tobacco industry would inevitably fund and conduct its own scientific research on its products, TobPRAC also emphasised the need for accompanying independent research and governance to effectively implement the framework.6
The ‘big 4’ transnational tobacco companies8 have since developed approaches to scientifically evaluate newer products which align with the recommendations of the WHO, IOM and TobPRAC. These companies publicise their scientific approaches and research via dedicated science websites (see External Links below).
Below is an outline of the approaches each transnational tobacco company states that it takes to scientifically assess its newer products. An overview is also provided showing the quantity of publications across each stage of each company’s scientific assessment approach. In the final section, some criticisms of the industry’s scientific approaches are summarised.
Philip Morris International
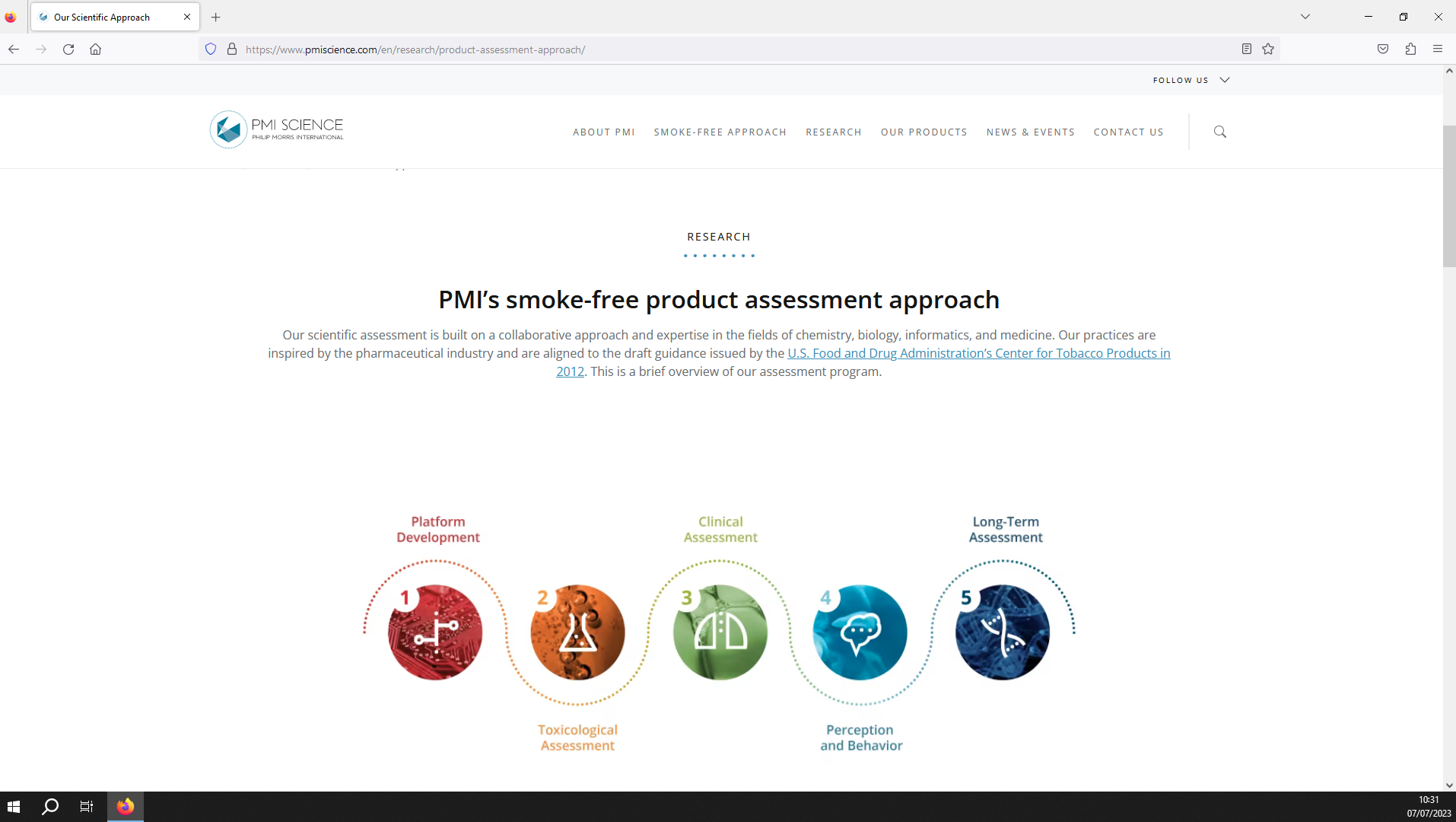
Image 2. PMI’s approach to scientifically assessing newer products.(Source: PMI Science website)9
Philip Morris International’s (PMI) scientific assessment approach has five stages (see image 2).9 The first stage, “product development”, comprises design and aerosol testing of potential newer products. The aerosol analyses aim to determine the physical and chemical properties of product emissions, which indicate the potential risks of the product.
Next, in vitro and in vivo “toxicological assessments” are used to measure the impact of the product emissions on cells and animals.
If this stage indicates that the product has reduced risk potential, the health effects of the products are tested in human users under controlled conditions (“clinical assessment”).
Following this, “perception and behavior” studies investigate consumer’s perceptions of the product, as well as user behaviours and levels of satisfaction.
Finally, in its “long-term assessment”, PMI states it will continue to monitor the biological effects and consumer acceptance of the product via safety surveillance, clinical studies, and epidemiological studies.9
PMI catalogues its scientific publications in a library on the PMI Science website.10 Publications held in this online library include: journal articles, presentations, posters, books, clinical trial registrations, posters, dossiers, reports, data, and methods and protocol documents. Up until May 2022, PMI assigned each publication in its library a tag relating to the relevant stage of its assessment approach. In addition to the five stages described above, two additional tags were used in PMI’s library: ‘Plant Biology’ and ‘Overview’. The number of publications assigned each tag (as of May 2022)11 is shown in Figure 1.
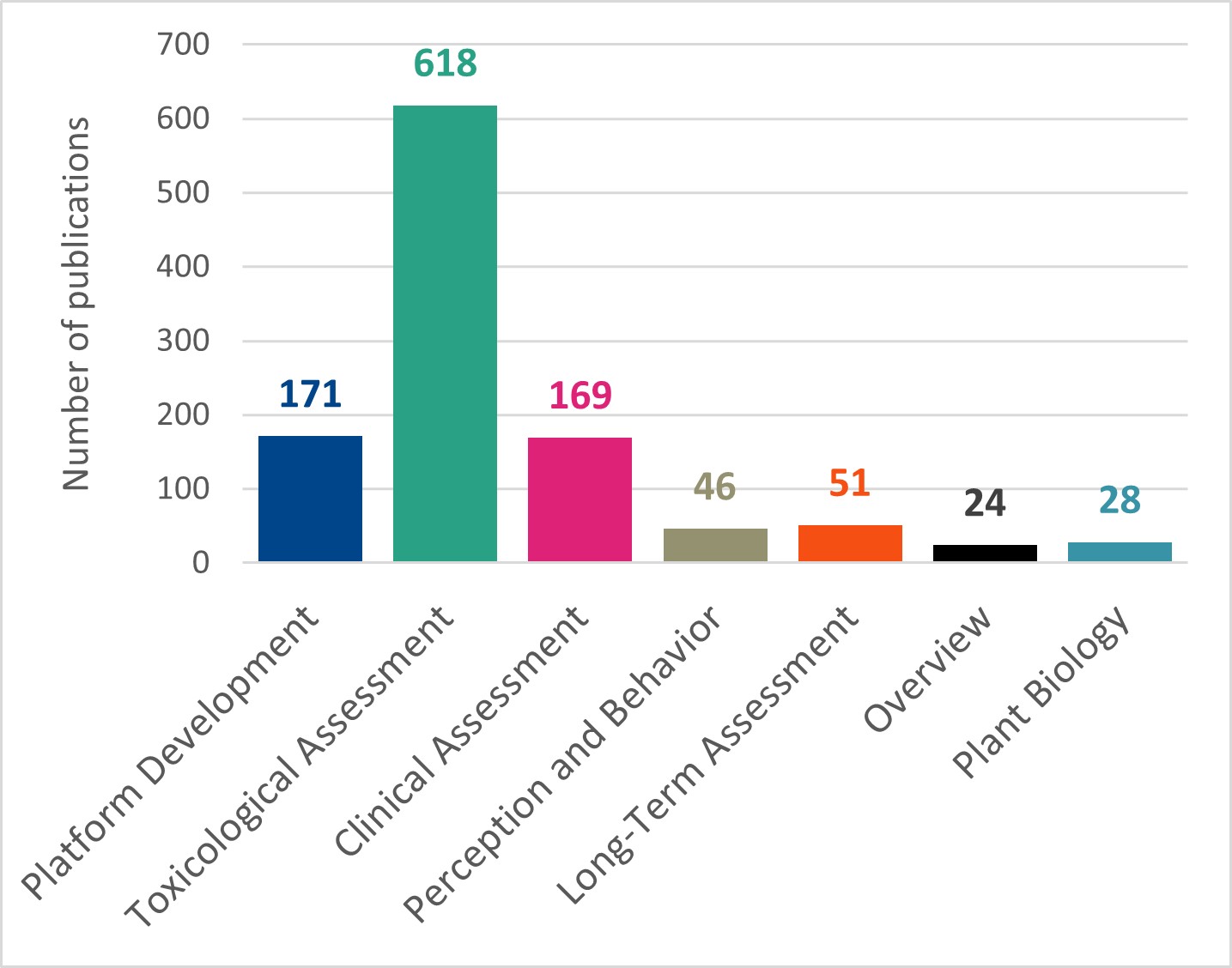
Figure 1. Number of publications tagged with each assessment stage in the PMI Science library as of May 2022. N.B. a single publication can have multiple tags.
British American Tobacco
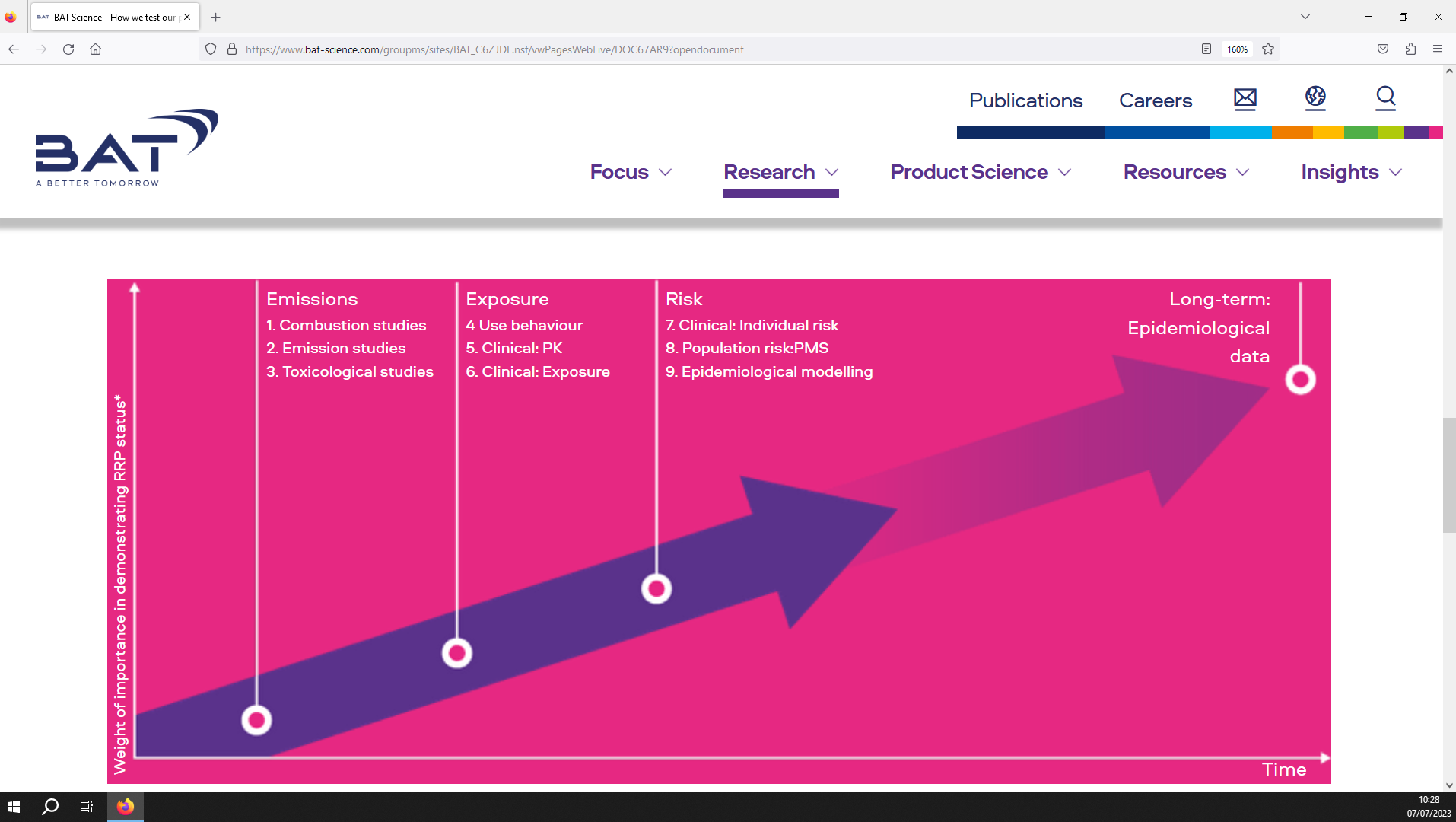
Image 3. BAT’s approach to scientifically assessing newer products.(Source: BAT Science website)12
British American Tobacco’s (BAT) scientific assessment approach consists of four stages (see image 3),12 fewer than the other transnationals. The preliminary “emissions” stage consists of studies which investigate whether the newer product functions correctly and whether combustion occurs (combustion studies), the chemical properties of the product emissions (emission studies), and then the effects of the product in vitro (toxicological studies).
Next, the product’s effects in human users are investigated. BAT states that “exposure”-related outcomes are assessed via clinical studies which analyse the behaviours exhibited by users (use behaviour), the short-term pharmacological effects of the nicotine in users (clinical: PK), and the levels of biomarkers of potentially harmful chemicals in users (clinical: exposure). “Risk” is assessed via medium-term clinical trials measuring the levels of biomarkers linked to harm and disease (clinical: individual risk), post marketing surveillance surveys to monitor the use of the product by consumers (population risk: PMS), and epidemiological modelling simulating potential impacts of the product on public health.
Finally, BAT states that it will use “long-term epidemiological data” to determine how the product is being used, as well as impacts on disease prevalence and public health.12
BAT catalogues its scientific publications in a library on the BAT Science website.13 Publications held in this online library include: abstracts, method documents, posters, presentations, journal articles, and ‘other’ publications.
Unlike the other transnationals, BAT does not categorise or tag the publications in this library by assessment stage.
Japan Tobacco International
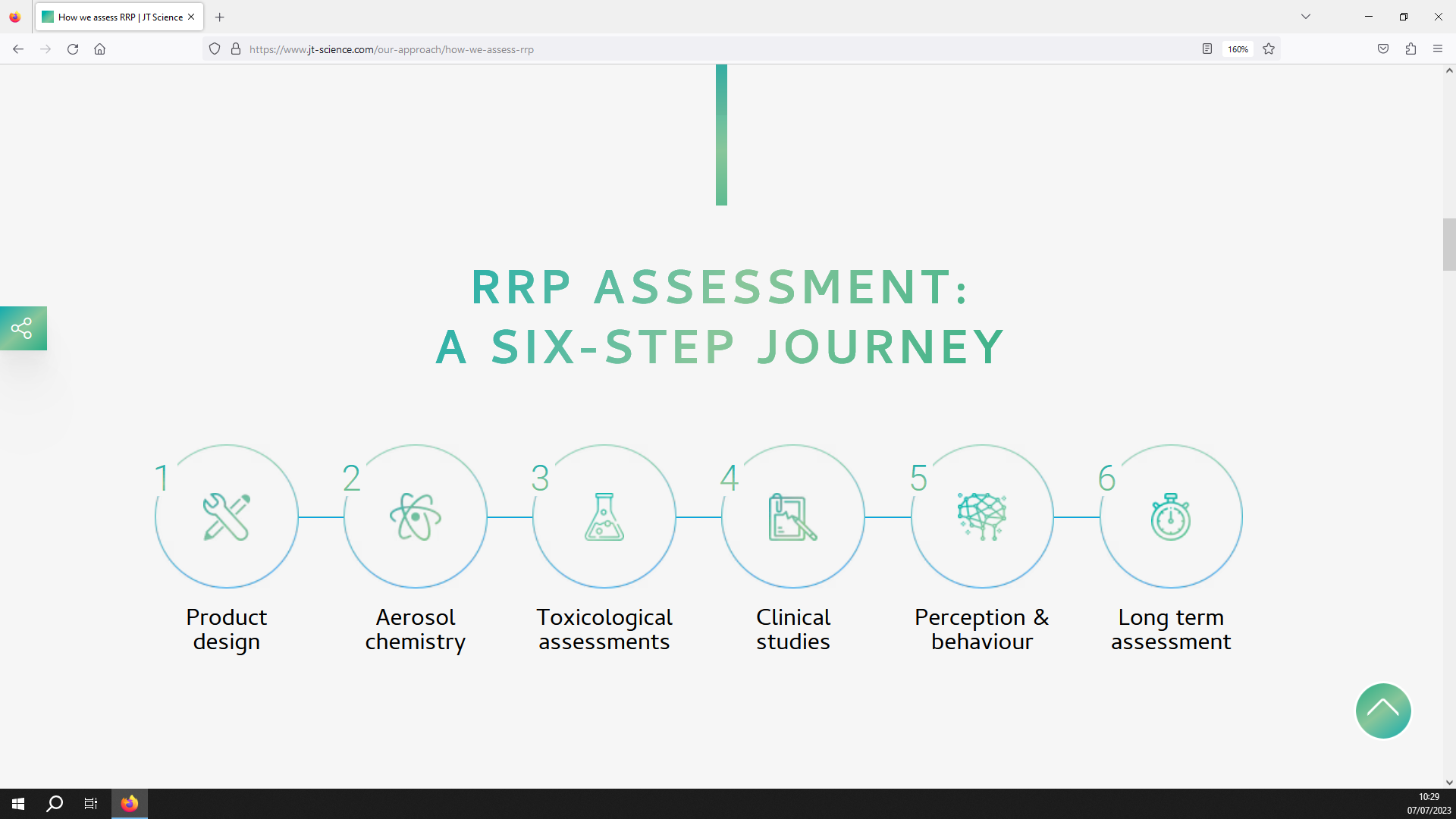
Image 4. JTI’s approach to scientifically assessing newer products. (Source: JTI Science website)14
Japan Tobacco International’s (JTI) scientific assessment approach consists of 6 stages (see image 4),14 the highest number of the four companies. JTI start with testing the design of its prototype products (“product design”) and the chemical properties of the product’s emissions (“aerosol chemistry”).
Next, in vitro and in vivo “toxicological assessments”, as well as in silico simulation studies, are used to assess the toxicity of the product.
“Clinical studies” will then investigate the effects of the product on human users, including risk reduction and pharmacokinetic studies.
The users experience, including satisfaction and patterns of use, are assessed through “perception and behavior” studies.
JTI state that it completes its assessment by monitoring the long-term effects of the newer product (“long-term assessment”).14
JTI catalogues its scientific publications in a library on the JTI Science website.15 Publications held in this online library include: journal articles, reports, posters, presentations, booklets, press releases, leaflets and news items. JTI assigns each publication in its library a tag relating to the relevant stage of its assessment approach. In addition to the six stages described above, two additional tags were used in JTI’s library: ‘Indoor Air Quality’ and ‘Other’. The number of publications (as of January 2023)16 assigned each tag is shown in Figure 2.
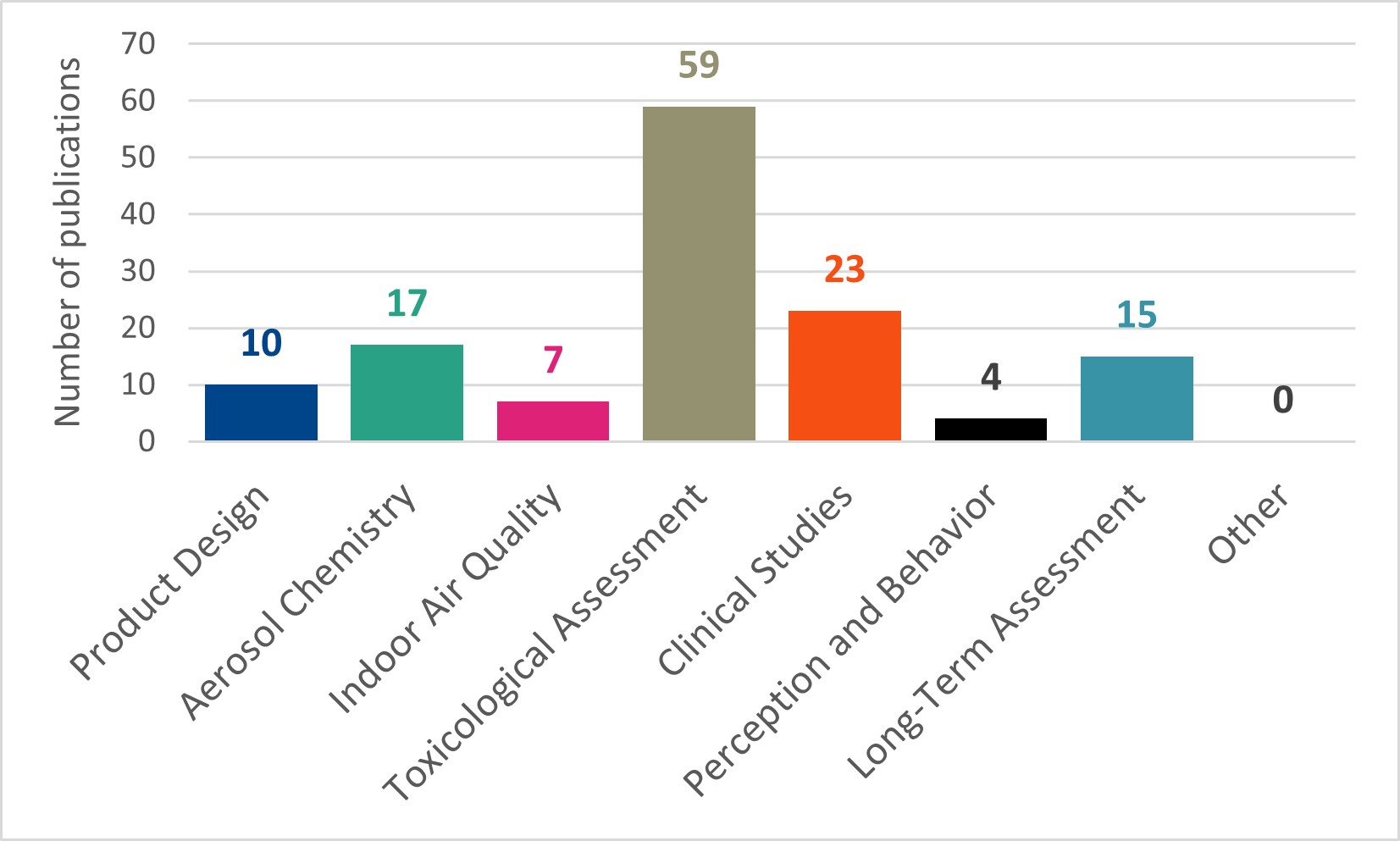
Figure 2. Number of publications tagged with each assessment stage in the JTI Science library as of January 2023. N.B. a single publication can have multiple tags.
Imperial Brands
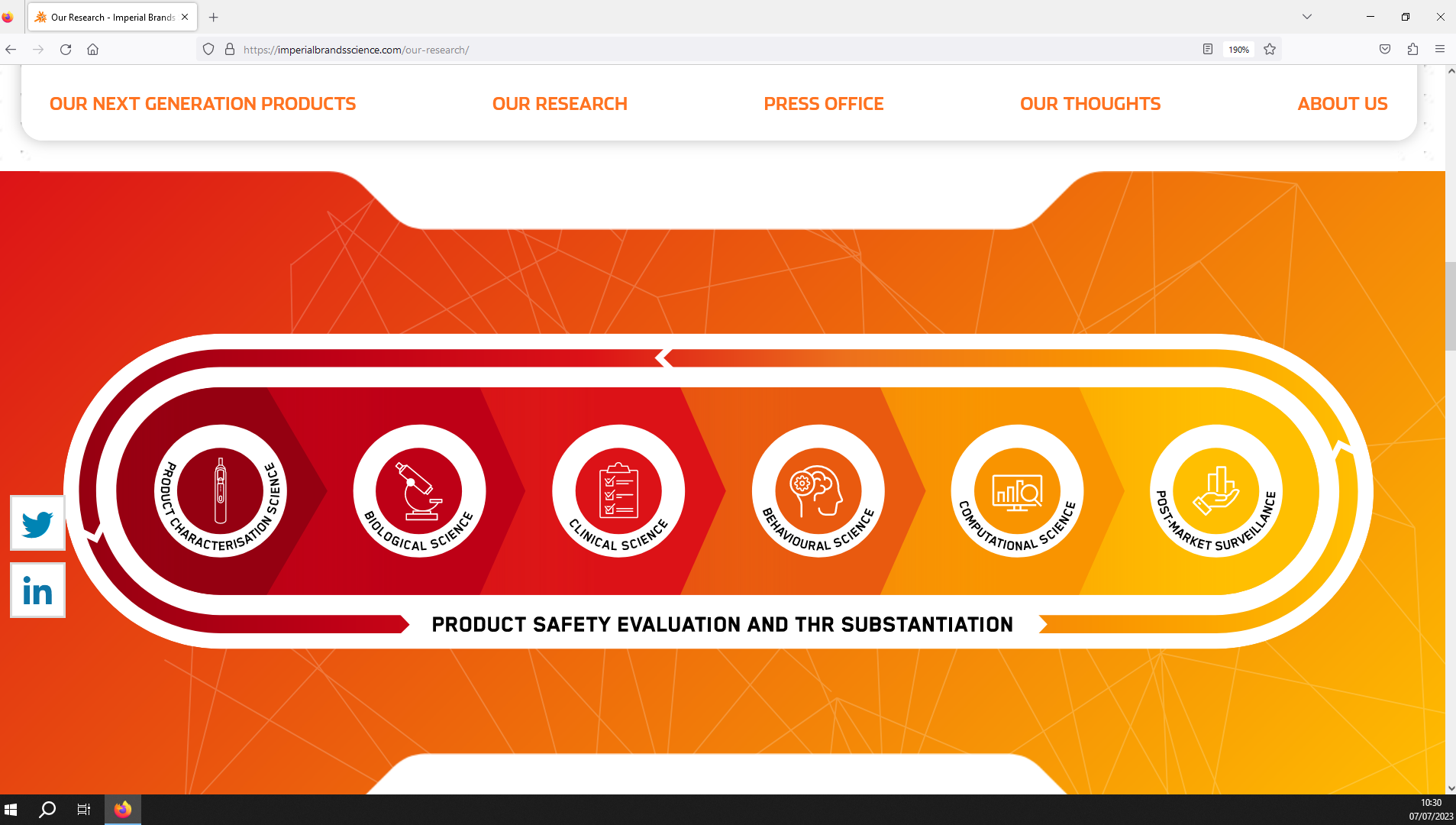
Image 5. Imperial Brand’s approach to scientifically assessing newer products.(Source: Imperial Brands Science website)17
Imperial Brands’ scientific assessment approach consists of 6 stages (see image 5).17 It begins with assessing the product’s design and emissions via chemical analyses and preliminary toxicological studies (“product characterisation science”).
Once this has been completed, Imperial Brands moves onto further “biological science”, including in vitro assessments to test the product’s toxicity to human cells.
Then, clinical studies are used to confirm that the reduced risk potential observed in the laboratory-based studies are also observed in actual human users of newer products (“clinical science”).
The “behavioural science” stage comprise studies to investigate the use, perceptions and addictiveness of newer products, both pre- and post-commercialisation.
In the next stage, “computational science”, comprise mathematical and computational modelling to estimate population-level use and health impacts.
Finally, Imperial Brands states that it conducts post-market studies measuring product use, user attitudes, adverse events, health-related outcomes and sales data (“population health science”).17
Imperial Brands catalogues its scientific publications in a library on the Imperial Brands Science website.18 Publications held in this online library include: journal articles, infographics, posters, presentations and videos. Rather than using tags that describe each stage of its assessment approach, Imperial Brands assigns each publication in its library one or more of the following tags: ‘pre-clinical’, ‘clinical’ and ‘post-market’. The number of publications assigned each tag (as of January 2023)19 is shown in Figure 3.
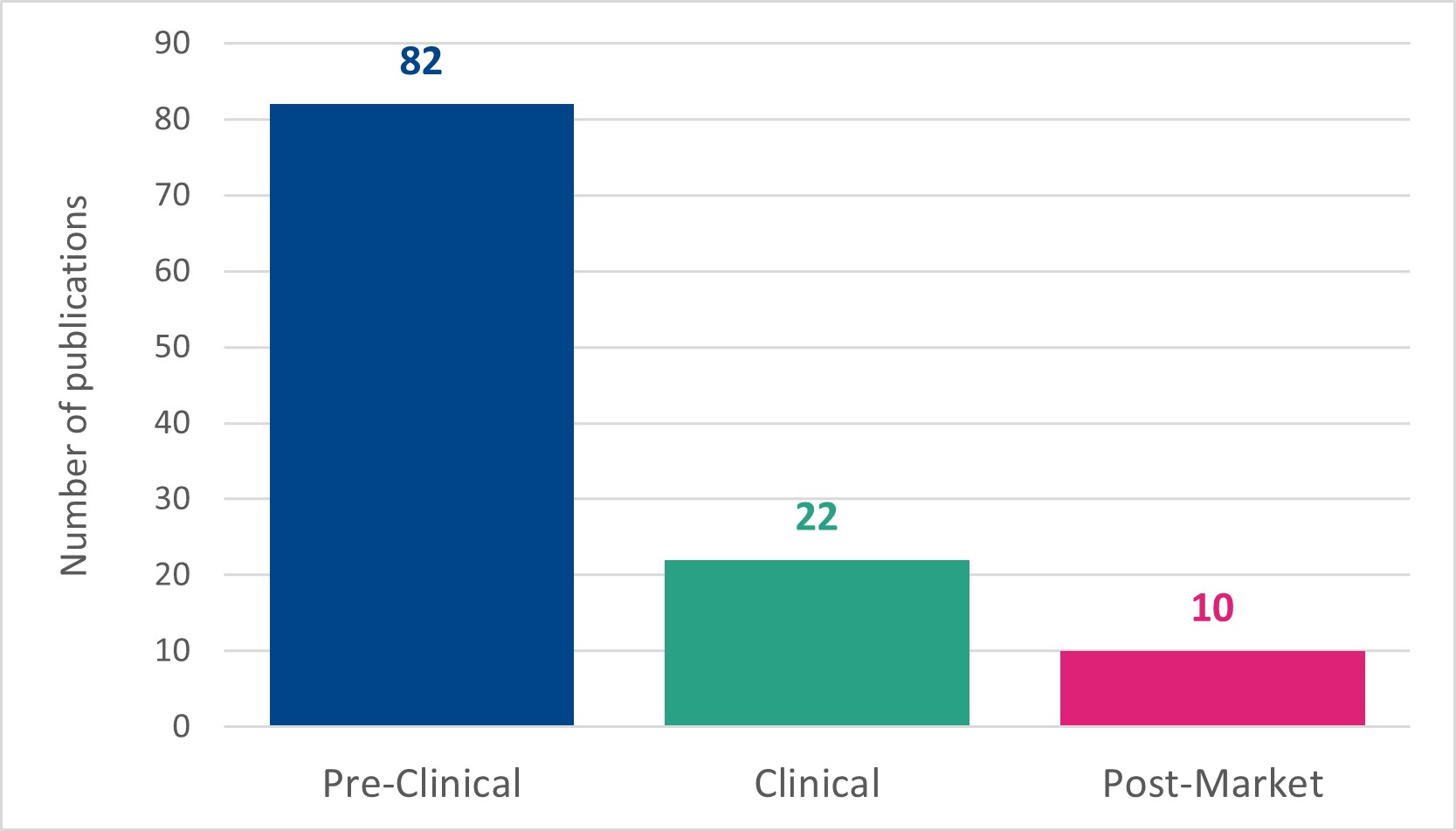
Figure 3. Number of publications tagged with each assessment stage in the Imperial Brands Science library as of January 2023. N.B. a single publication can have multiple tags.
Criticisms of the Industry’s Approaches
The tactics the tobacco industry uses to influence scientific research in order to further its economic interests are well documented.202122
- For more details see Influencing Science
There is evidence that the industry continues to employ strategies to influence research and its approaches do not prevent bias. For example, the tobacco industry, including PMI and BAT, has used external scientific consultants23 and publishes its research in journals with which it has ties.24 Such strategies help to influence the volume, credibility, reach and use of science.25
The industry has a history of designing clinical research to substantiate and develop harm reduction claims, especially those around reduced exposure to harmful chemicals compared to cigarettes.26 A 2022 systematic review critically assessing clinical trials on HTPs, most of which have been conducted by tobacco companies, fall short of what is needed to adequately investigate whether HTPs were beneficial to public health.27 The same review found most of the industry’s clinical trials on HTPs were at high risk of bias, particularly due to inadequate blinding of participants (concealing information from participants that might influence results)28 and selectively reporting results.27 A systematic review of the e-cigarette literature found studies by independent authors were more likely to report potentially harmful effects of e-cigarettes, while the majority of studies by tobacco, e-cigarette and pharmaceutical companies reported no harmful effects.29
All the transnational companies include epidemiological or long-term studies in their assessment approaches. However, according to each company’s Science webpages,14303132 their own publication library tags (see Figures 1-3), and reviews of both the HTP333435 and e-cigarette literature,3637 it appears that, to date, the industry has conducted few epidemiological or long-term studies.
As noted by TobPRAC, Article 5.3 of the WHO FCTC calls for regulatory decisions on tobacco products and scientific assessment of tobacco products to be made independent of the tobacco industry.6
For more details see FCTC Regulations on the Need to Protect Public Health Policies from Tobacco Industry Interference