Foundation for a Smoke-Free World’s Tobacco Transformation Index
This page was last edited on at
Issue at a Glance
- The “Tobacco Transformation Index” (TTI) is a project of the Foundation for a Smoke-Free World (FSFW). It describes itself as an “objective” index for measuring the corporate transformation of the tobacco industry.12
- However, FSFW is wholly-funded by Philip Morris International (PMI), the second-largest transnational tobacco company in the world.
- The lack of independence and external oversight, extensive industry involvement and flawed methodology of the TTI produces a fundamental misalignment with public health and tobacco control goals. The TTI instead enables industry self-promotion and furthers tobacco companies’ corporate goal of profit maximisation.
Background
In September 2017, FSFW was established using a US$80 million investment from PMI, announced as the first of twelve annual instalments totalling US$960 million.34 Though FSFW initially claimed that it was “seeking and expects to receive funding from other sources” in addition to PMI,5 in June 2020 the wording on the funding page of its website changed to “may seek funding from other sources”.6
FSFW has divided its research “core work pillars” into three streams: Agriculture & Livelihoods; Health, Science & Technology; and Industry Transformation.7 Based on FSFW’s Mid-Point Strategy Update documents published in July 2020, which FSFW states assess its progress, mission alignment and cohesiveness halfway through its 2019-2021 Strategic Plan, it appears that aside from a single grant to a UK-based company on FSFW engagement with China (Feng Insight: US$220,638), the sole product of the Industry Transformation workstream is the TTI.8
The original Request for Proposals (RfP) issued by FSFW in October 2018 called TTI the “Smoke-Free Index”.910 However, the Southeast Asia Tobacco Control Alliance (SEATCA) has published a Smoke-Free Index since 2016. SEATCA’s Smoke-Free Index was developed to assess the extent to which smoke-free policies in the Association of Southeast Asian Nations (ASEAN) align with the WHO FCTC. In July 2019, SEATCA posted an official complaint on its website with a statement from its Executive Director, Dr Ulysses Dorotheo to say that “[the Foundation’s] use and trademarking of the term “Smoke-Free Index” is misleading, potentially confusing, and tantamount to wrongful appropriation of SEATCA’s intellectual property”.11 FSFW changed the index’s name to the “Tobacco Transformation Index” thereafter, but without public reference to its appropriation of the name of SEATCA’s index.
In March 2019, two grantees under the industry transformation work pillar were announced: market research company Euromonitor International and a private research consultancy SustainAbility.12 According to FSFW’s 2019 tax return, it gave US$2,005,158 in funding to Euromonitor in 2019, with US$4,524,534 committed for the future. SustainAbility received US$3,127,420, with a further US$1,820,503 agreed.13 Euromonitor has also received funding from PMI for its work on PMI IMPACT, (with Euromonitor’s announcements of these funding streams coming only a day apart)14 and was awarded a second grant by FSFW in March 2020 to analyse the impact of the EU menthol ban.8 In January 2020, Biochromex was awarded US$29,100 to produce a “Nicotine Product Relative Risk Assessment”.1516 This assessment was published on 16 September 2020 in a pre-print repository, without peer review (more below).17 On FSFW’s grants webpage, this work is classified as “Industry Transformation”, but on its Mid-Point Strategy document, it is classified as “HST+”, a designation used to indicate education and awareness associated with the Health, Science and Technology research pillar.8
In 2020, a new website was created specifically for the Tobacco Transformation Index: https://tobaccotransformationindex.org/. There, it detailed TTI’s premise, design and management and governance, including the formation of an “External Advisory Panel”. FSFW is not mentioned on the homepage of the website. Over the course of 2020, it released several documents through this website:
- External Advisory Panel Charter (undated)
- Stakeholder Consultation Report (March 2020)
- Industry Consultation Summary (May 2020)
- Response to Industry Feedback (May 2020)
- Preliminary Index Methodology (May 2020)
- Preliminary Country Indicators (May 2020)
Upon the TTI’s final release in September 2020, the following documents were also added, as well as a webpage detailing the final company rankings:18
- 2020 Index Ranking Report: Executive Summary (September 2020)
- 2020 Index Ranking Report (September 2020)
- 2020 Index Methodology (September 2020)
- Advisory Panel Statement on 2020 Index (September 2020)
The planned Country Fact Sheets are listed as “coming soon” on the website’s “Methodology” webpage.19
Lack of independence
As an organisation wholly funded by the tobacco industry, Foundation for a Smoke-Free World (FSFW) is neither an objective nor independent research body. Previous critiques have shown how FSFW has an unavoidable conflict of interest in producing research and advocacy on the tobacco industry: you can find them outlined at Foundation for a Smoke-Free World: How It Frames Itself.
The Tobacco Transformation Index (TTI) is, in its own words, “convened, funded, and overseen” by FSFW.20 On the TTI website, FSFW is described as “responsible for overall strategic direction and governance of the Index”20 and the party with “ultimate accountability and decision-making”.21
The same critique of FSFW as not independent applies to TTI: as a project funded by an organisation funded solely by the tobacco industry, it cannot be considered objective or legitimate.
In its own Stakeholder Consultation Report, participants in consultations on TTI (facilitated by FSFW grantee SustainAbility) shared this concern about its legitimacy as a product of FSFW, and thus, tobacco money: “many participants felt that [FSFW funding independence policies] do not sufficiently mitigate the appearance of a dangerous conflict of interest”. FSFW concluded this report with a recommendation to “spin out” future versions of TTI as “an independent entity with a fiduciary board”.21 After the release of the final 2020 Index report, FSFW confirmed on the TTI website that it would “evaluate alternative governance structures, including funding mechanisms, to maximize the impact of the Index on its purpose for the long term”.22
FSFW has previously made promises of seeking alternative funding for itself that it later reversed (as detailed above). No additional funding partners have been announced for TTI as of September 2020. Even if TTI were transferred to another governance structure , it would still remain an industry-influenced product due to its original funding and design.
Lack of public health involvement
Although the External Advisory Panel Charter says that members should include those with expertise in “public health and tobacco control”,23 the panel members as of September 2020 have no experience in tobacco control.24 This may indicate that FSFW struggled to appoint tobacco control experts to the panel.
Two stakeholder consultations on TTI were cancelled in Thailand and Turkey after pressure from policymakers and academics. In Turkey, the event was cancelled by the government, and in Thailand, by SustainAbility itself, due to a lack of interest by potential attendees and amidst reports that the academic community would boycott the event.21
Lacks external oversight
As discussed above, FSFW maintains ultimate governance over TTI, despite its claims of independence.24
Neither the data nor methodology are independently verified,2 despite recommendations from stakeholder consultations that both an external validation and technical advisory board be established. FSFW says it will seek independent verification of company data in future.2125
Engagement with industry representatives is also inadequately documented. The minutes provided by FSFW on the TTI website of consultation calls between Euromonitor and Philip Morris International (PMI), British American Tobacco (BAT) and Swedish Match are very brief.26 Additionally, company responses to the initial consultation letter in January and February 2020 were not published.
Industry heavily involved in development
The TTI embraces as its premise that, through “constructive engagement, investors and other stakeholders can more clearly articulate their expectations and influence companies to change”.27 Historical and contemporary evidence, however, shows that the tobacco industry has consistently used engagement with government and third parties to deceive the public about the perils of using its products and promote its own interests, to the detriment of public health.
Beginning in the late 1980s and continuing into the 2000s, Philip Morris International funded scientists to “restore the acceptability of smoking” and “sustain controversy” about the harms of secondhand smoke through The Whitecoat Project. Evidence from these industry-funded third parties was used to “resist and roll back smoking restrictions” around the world.28
More recently, the Global Tobacco Industry Interference Index documented global attempts by the tobacco industry to influence policymakers. In Japan, the country with the worst ranking in 2019, the close relationship between the government and Japan Tobacco Group (parent company of Japan Tobacco International) led to the dismissal of both smoke-free public places draft legislation in 2017 and a proposal for pictorial health warnings on packaging in late 2018.29
FSFW said in its initial Request for Proposals (RfP): “there will be no consultation with industry employees regarding the Index-making process, which comprises index development, compilation, scoring, and reporting”.9
Yet in the introduction to its Industry Consultation document, it is clear that the industry was heavily involved in the creation of TTI. Euromonitor, which was tasked with TTI design, data analysis and final scoring, was also the grantee in charge of industry consultation. For each phase of development, including index design, methodology, formal data submission and data validation, companies were “given the opportunity to review, comment and relay feedback”:26
“Multiple touch points across these four phases and extensive communication with updates throughout this iterative process, help to reinforce a collaborative approach to Index development…Apart from written feedback, the Index team has also offered conference calls to provide companies with the opportunity to share more details on their feedback, ask questions directly to the Index team and have a productive conversation.”
In the final TTI report, data informing assessment included “interviews with industry experts”.2 These “industry experts” are further defined as “trade organizations, former company employees, and relevant stakeholders across the supply chain”.30 It is unclear whether this group was included in the “expert review” phase of finalising the methodology.30
Part of the “data validation” phase is the referral back to companies to “review data informing preliminary score and propose clarification of any discrepancies”.26 Given no external data verification is included in this phase, it is difficult to see how this step allows for any more than tobacco companies to dispute any poor scores they may receive.
Tobacco industry suggestions for additions and alterations to the types of data to be gathered, detailed in the Industry Consultation report, also appear in the Preliminary Methodology. As the version of the methodology that was sent to companies in November 2019, according to FSFW,26 is not publicly available, it is not possible to conclude the extent to which industry suggestions influenced the final TTI methodology.
Industry feedback that appears in the methodology includes:3031
- Swedish style moist snuff (known as “snus”) be reclassified as reduced-risk and differentiated from Asian-style chewing tobacco (recommended by Swedish Match)
- Incorporation of a metric on illicit trade (recommended by BAT): TTI does not include a metric on illicit, but does incorporate it into the associated Preliminary Country Indicators.
- Ratio of marketing spend of “high-risk” to “low-risk” products (general)
- Capital allocation to include research and development (R&D) and capital expenditure (general)
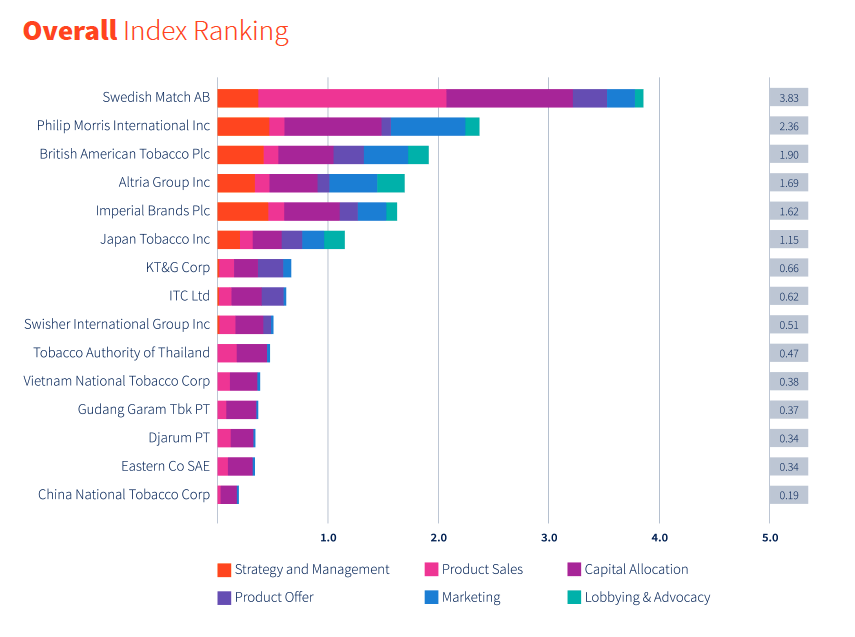
Image 1: The final ranking of the 2020 Tobacco Transformation Index. Note the top six companies are publicly-traded transnational tobacco companies. (source: TTI 2020 Index Report)
The three companies that provided additional data to Euromonitor on follow-up calls were ranked top three in the final TTI ranking (Image 1).226 Despite the industry’s heavy involvement in the development of TTI, there is little indication to suggest that tobacco companies submitted their own data in addition to publicly available information or existing databases, including Euromonitor’s own. One of the limitations included in the final report was the “lack of data availability”.2
Misrepresents tobacco control and harm reduction
The Tobacco Transformation Index (TTI) positions tobacco harm reduction as necessary to aid “failing” tobacco control. This is a false narrative; tobacco control is successful worldwide.
The Cancer Prevention & Control Program at Georgetown University, USA estimated that implementation of WHO FCTC MPOWER measures in 2007 would result in 22 million fewer projected smoking-attributable deaths by 2014.32 ‘Conventional’ tobacco control policies such as price and tax policies; comprehensive smoke-free policies; health warnings; bans on tobacco advertising, sponsorship and promotion (TAPS); and evidence-based cessation interventions are consistently found to be the most effective measures to reduce tobacco-related health harms and encourage quitting. 33 One of the single largest barriers to effective policy implementation is tobacco industry interference.33
Tobacco harm reduction is promoted by industry & allies as ‘the solution’, when in reality, it is only effective as a public health activity when other strong tobacco control measures are in already in place. Research by members of the Tobacco Control Research Group (TCRG) has found that the industry adopts harm reduction as “opportunistic tactical adaption to policy change rather than a genuine commitment to harm reduction”.34
A common narrative used to bolster the industry’s claims on harm reduction is the ‘Swedish Experience‘: that Sweden experiences low rates of smoking and tobacco-related disease as a result of the presence of Swedish-style moist snuff, or “snus”, being available in the country. While snus may cause less harm to users than combustible cigarettes, many commentators have argued that Swedish data are a result of strong tobacco control legislation, rather than attributable solely or primarily to snus.35 This “proof of concept” idea has been promoted by another FSFW grantee, Knowledge-Action-Change.36
While the use of newer nicotine and tobacco products may reduce harm when compared to the use of combustible cigarettes, the technical basis of arguments around the relative risk of newer products in the development of the TTI is very limited. This is similar to the way in which US FDA’s July 2020 decision to issue modified exposure status to its HTP, IQOS, risks conflating HTPs and e-cigarettes creating confusion among the public, and even governments.37 In its Response to Company Feedback, FSFW stated that its classification of products as “reduced-risk” and “high-risk” (Image 2) in its preliminary methods document is based on only two papers, by Nutt et al. and Abrams et al. (Image 3 and 4). It uses this same classification in the final TTI, based on a non-peer reviewed, pre-print analysis commissioned by FSFW itself.17231383940
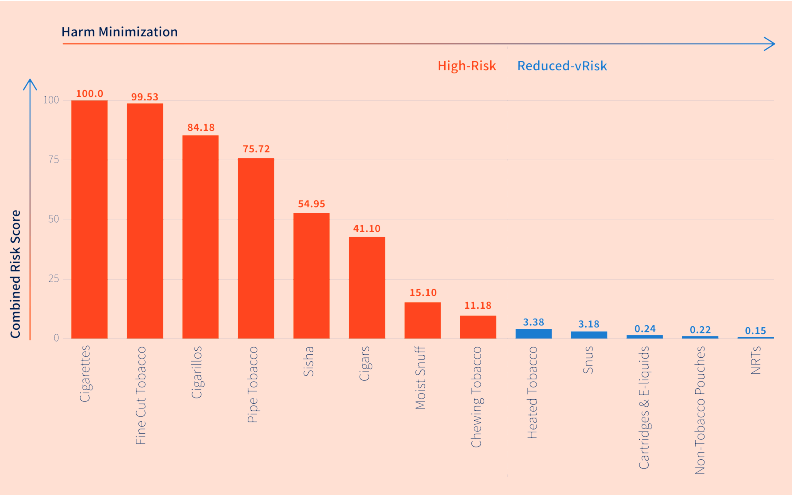
Image 2: TTI classifies “high-risk” products as including all traditional combustible tobacco and chewing tobacco products; “reduced risk alternatives” include e-cigarettes, heated tobacco, moist snuff (aka “snus”), nicotine replacement therapy and nicotine pouches. This image is drawn from the pre-print paper commissioned by FSFW from Biochromex (source: TTI 2020 Index Report)
Preliminary nicotine product characterisation based on two publications
Nutt et al.’s 2014 paper aimed to estimate the relative harms of nicotine-containing products based on the opinions of an expert panel (Image 3). The authors themselves flag the following limitations of the paper:40
- “a limitation of this study is the lack of hard evidence for the harms of most products on most of the criteria” (i.e. the scorings are based on “knowledge and experience alone”) and
- “there was no formal criterion for the recruitment of the experts”.
An editorial in The Lancet describes the evidence in the paper as “extraordinarily flimsy”, since “the opinions of a small group of individuals with no prespecified expertise in tobacco control were based on an almost total absence of evidence of harm”.41
The paper acknowledges funding from EuroSwiss Health and ‘support’ from Lega Italiana Anti Fumo (LIAF).40 EuroSwiss Health was founded by Delon Human, who had previously received funding from British American Tobacco (BAT). In 2013, he endorsed BAT’s public health credentials in the company’s sustainability report.424344 The chief scientific adviser (previously President) of LIAF is Riccardo Polosa, who has received funding from PMI, PM USA and FSFW; and has worked as a contractor for BAT.45
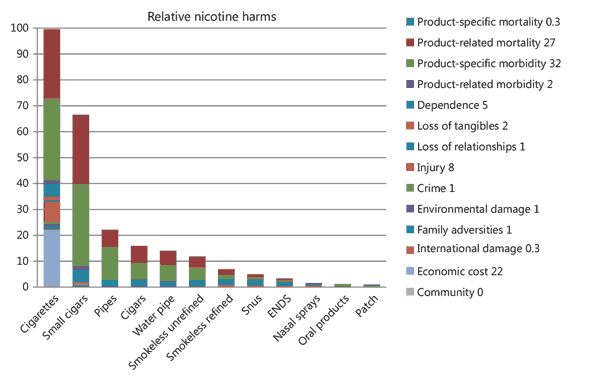
Image 3: Figure from Nutt et al., 2014 that categories tobacco and nicotine products by relative harms. (source: Nutt et. al, 2014)
The second paper on which the TTI bases its interpretation of high/reduced risk categories of newer products in its preliminary documents is Abrams et al., 2018. The ‘harm minimization continuum’ developed in this paper (Image 4) is adapted from Nutt et al.’s paper, which as outlined above, based its findings on the ‘knowledge and experience’ of a small group of individuals.
The authors use the term ANDS (“alternative nicotine delivery systems”) to describe products that do not combust tobacco, and state that this encompasses both e-cigarettes and ‘heat-not-burn’ tobacco. This description is in contrast to the first independent study on PMI’s heated tobacco product, IQOS, which, in 2017, found that heated tobacco products produce harmful chemicals similar to smoke through pyrolysis (incomplete combustion), just as in combustible cigarettes. The authors stated that calling these products “heat-not-burn” are “dancing around the definition of smoke”.46
Abrams et al. do flag the limitations of the existing evidence base on the relative risk of newer products, saying “new and evolving ANDS products may raise new issues and data needs. For example, products that heat rather than burn tobacco, but still mimic smoking, may raise issues different from those raised by e-cigarettes.” As such, similarly to Nutt et al’s paper, this paper does not attempt to synthesise the evidence base on the potential harms of HTPs.39
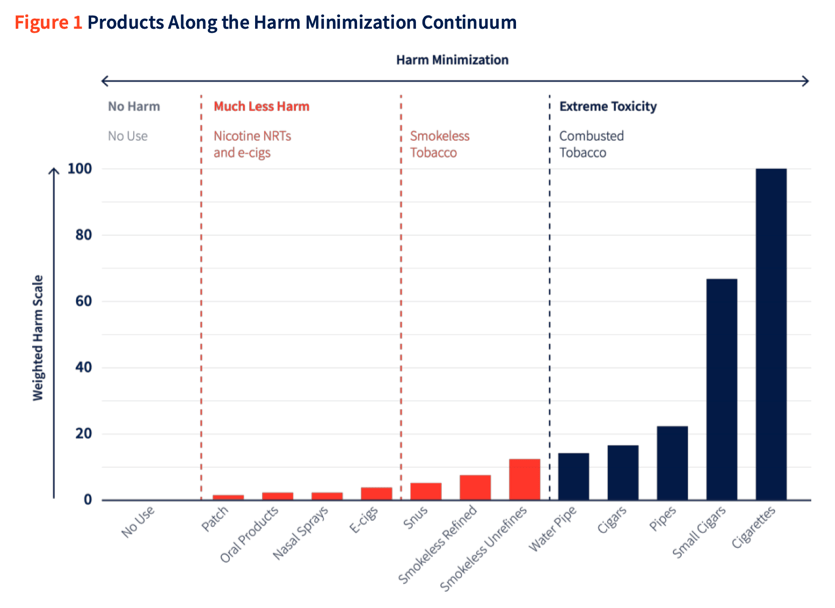
Image 4: The ‘harm minimisation continuum’ developed by Abrams et al. (source: TTI Response to Company Feedback)
Its authors include David Abrams and Raymond Niaura, who, In January 2020, were both named to FSFW’s scientific advisory board.47 Both have previously appeared as speakers at the Global Tobacco and Nicotine Forum (GTNF), an industry-sponsored event,48 and received funding from e-cigarette manufacturer Cuts Ice e-Liquid Laboratories for “publication and open access costs” (which was not declared as a conflict of interest).49
TTI classification of products by risk based on FSFW-funded paper
While the TTI’s preliminary documents base their classification of the relative risk of nicotine products on these two papers discussed above, for the TTI’s final product, FSFW commissioned a further review on which to base the index’s classification.
FSFW grantee, Biochromex, was paid US$29,100 in 2020 by the Foundation for a “Nicotine Product Relative Risk Assessment”.1516 Biochromex is a research services company which has worked with the healthcare, pharmaceutical and consumer goods industries. It provides ‘tailored, systematic literature review, competitive landscaping and content writing’ for its clients50 and since February 2017 its director has been Dr. Rachel Murkett.51
On 16 September 2020, Biochromex authors (including Murkett) released a paper entitled ‘Nicotine Products Relative Risk Assessment: A Systematic Review and Meta-Analysis’ on the online platform, OSF Preprints.17 This output is a non-peer-reviewed publication, and the pre-print platform seemingly does not allow for reviews of the paper, unlike many other pre-print platforms. Although the paper declares funding from FSFW (with no mention of FSFW being fully-funded by PMI), its authors assert they have no conflicts of interest.
The paper is a systematic review and meta-analysis, used to create a “relative risk hierarchy” of 13 nicotine products. The paper’s introduction relies heavily on the Nutt et al and Abrams et al papers outlined above, saying “these studies represent an excellent foundation upon which the data-driven assessment of the relative risk of nicotine products can be built”.17
The TTI uses this paper to classify nicotine products into high risk (which includes combustible cigarettes, cigarillos, pipe tobacco, shisha, cigars, moist snuff and chewing tobacco) and ‘reduced risk’ (which includes heated tobacco products, snus, e-cigarettes and nicotine replacement therapy; Image 2). The TTI also uses the relative risk scores calculated within Murkett et al’s paper to calculate the scores within ten of its 35 metrics, saying in its “Approach to Scoring” section:30
“The relative risk assessment…was leveraged to evaluate companies’ offerings and sales of reduced-risk products, and to further compare these according to their relative risk. To do so, the relative risk by product was used as a multiplier for each company’s sales and offer in the high-risk and reduced-risk categories. For example, volume sale of cigarettes was multiplied by the relative risk of cigarettes (100), while volume sale of chewing tobacco was multiplied by its relative risk (11.18), to reflect the different level of risk associated with each type of product.”
However, key steps of Murkett et al.’s methodological procedure are opaque. The paper fails to list the 320 studies on which its analysis is based. It also fails to report the funding sources of these studies, meaning it is impossible to tell the extent to which the paper relies on tobacco industry funded science. The authors themselves concede that one of the paper’s limitations is “the lack of comprehensive and high-quality data, which leaves significant gaps in the analysis”.17
In fact, the order in which HTPs are located on the “relative risk hierarchy” reproduced in the TTI (below chewing tobacco, in the “reduced risk” category) is based on a lack of data, according to Murkett e al.:17
“With the exception of snus, the reduced risk products are not represented at all in the epidemiological analysis, which can be attributed to their relative novelty compared with combustible and smokeless tobacco. In the toxin emissions analysis, the heat-not-burn devices place higher than chewing and dipping tobacco, however, this order is reversed in the final hierarchy due to the position of chewing and dipping tobacco relative to combustible cigarettes in the epidemiological analysis.”
Despite evidence that heated tobacco score higher in terms of toxin emissions than chewing tobacco, a “high-risk” product, it was moved to the “reduced risk” category without any epidemiological evidence to support this reordering. Thus, the TTI’s conceptualisation of a ‘reduced-risk’ product category, and calculations for ten of its metrics, are based on a paper which has several flaws. The TTI lists both heated tobacco products (HTPs) and e-cigarettes in the “reduced-risk” category, giving them implicit equivalence in their risk profile, despite a lack of consensus on the potential harms posed by HTPs.
Both the US Food and Drug Administration (FDA) concluded that the evidence submitted by PMI in support of HTPs was insufficient to merit a “reduced-risk” label (FDA)52535455 The Australian Therapeutic Goods Administration (TGA) reached a similar conclusion, and blocked the importation of HTPs into the country (TGA).5657
Alignment with industry corporate strategy
The “corporate transformation” narrative is essential to tobacco industry strategy; as its primary business model of selling combustible tobacco products begins to fail, it must turn to new products to maintain its profits and ensure its future existence.58 PMI in particular has used “transformation” language associated with its newer products in order to achieve a number of corporate goals, as revealed within its leaked corporate affairs plan from 2014. Here it outlined its plans to use harm reduction arguments to tackle industry “denormalization” and to “establish PMI as a trusted and indispensable partner”, in support of both its “combustible and reduced risk (RRP) product businesses”.5958
The TTI further supports this industry narrative, effectively rewarding transnational, publicly-traded tobacco companies for following their profit motive. The six publicly-traded tobacco companies were ranked top of TTI, despite their poor scores for the volume of high-risk products sold, one of the key indicators of real world transformation of their business. This was possible because of the additional points awarded for following corporate environmental, social and governance (ESG) frameworks, and the massive investments of money on research and development (R&D) which their profits allow them.2
In reality, however, the tobacco industry has not transformed. Instead of supporting tobacco control measures, including increased tobacco taxes, which would drastically reduce the harm caused by tobacco product use, the industry continues to vigorously oppose them.5829 The industry has not stopped selling cigarettes; tobacco kills more than 8 million people around the world each year 60 and cost US$1436 billion in global healthcare expenditure and productivity loss in 2012.61 It continues to launch new brands of combustibles, especially targeting low- and middle-income countries (LMIC), where tobacco control legislation may be less well enforced.586263 In fact, 40% of the cost of smoking is disproportionately incurred by LMICs.61
Like other sustainability indexes, the TTI does not evaluate industry business practices such as lobbying against evidence-based tobacco control measures,29 sourcing tobacco farmed using modern slavery and child labour,64 evading taxes65 and flouting advertising bans to market its products to youth.66 The TTI therefore legitimises tobacco companies’ efforts to portray themselves as responsible corporate citizens.
Despite FSFW statements that a ranking from “bad” to “less bad” will prevent tobacco companies from using TTI as a form of promotion and corporate social responsibility (CSR),21 the final TTI report reads as a scorecard; the External Advisory Panel confirms this in its statement, calling the TTI “a nuanced scorecard and benchmark”.25 We have already seen tobacco companies incorporating indexes and awards into their CSR and business strategies. See our page on CSR: Awards to find out more.
Appropriation of sustainable development
The Tobacco Transformation Index (TTI) says that it “aims to contribute to SDG 3 (Good Health and Well-being) and SDG 9 (Industry, Innovation, and Infrastructure”.3031 Yet, like the tobacco industry, FSFW also ignores the inclusion of Target 3A in the United Nations Sustainable Development Goals (SDGs). Target 3A calls for countries to “strengthen the implementation of the World Health Organization Framework Convention on Tobacco Control”, including Article 5.3 (non-engagement with the tobacco industry).67 The tobacco industry funding and close collaboration on the development of TTI directly contradict WHO FCTC and thus Target 3A.
- Read more about the tobacco industry’s appropriation of sustainable development on our Greenwashing
Flawed and inadequate metrics
This page does not seek to provide a comprehensive, in-depth criticism of the methodology of the Tobacco Transformation Index (TTI). However, there are several overarching problems with its preliminary methodology.
Inadequate theory of change
The TTI relies on a theory of change that shareholders will be able to apply pressure to companies to get them to change their behaviour. Regardless of the plausibility of this theory, nine of the 16 companies included in the 2020 TTI were private and state-owned companies. It is unclear how the TTI could be used to influence these companies. The Expert Advisory Panel noted:25
“The Index has identified institutional investors as a primary user of the Index, with the potential to actively engage with tobacco companies to transform the industry. In the case of private and state-owned companies, this lever is held by shareholders and governments that may have the luxury to ignore market and stakeholder pressure to transform the tobacco industry. The Index team, the Panel, and FSFW all acknowledge this poses an outsized challenge relative to the creation of other indexes.”
The Panel recommends that FSFW “urgently develop a subsidiary theory of change for the Index relevant for the non-publicly quoted companies”.25
Overemphasis on “commitment” and “transparency”
There is an overemphasis placed on metrics used to assess “commitment” to industry transformation. The 35 indicators of TTI are organised around three main “strategic pillars”: commitment, performance and transparency. FSFW describes the commitment pillar as an assessment of “the extent to which the company has incorporated the goal of industry transformation into its vision and strategy, its internal policies and codes of conduct, and its public stance and subsequent action”.30
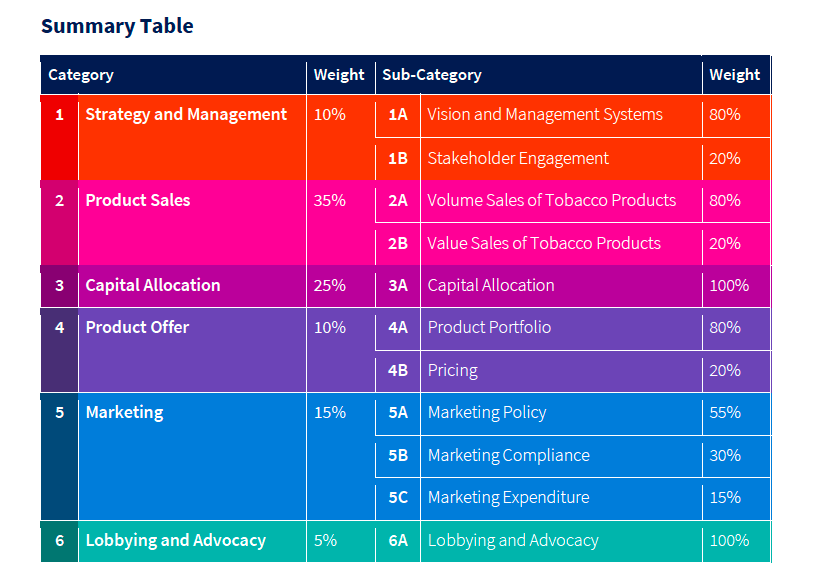
Image 5: The weighting of TTI indicators according to its methodology. Note the cumulative weight of commitment and transparency indicators (strategy and management, marketing, lobbying and advocacy; 30%) is roughly equivalent to that afforded to product sales (35%). (source: TTI 2020 Index Report)
This issue is reflected in the way that metrics are weighted (Image 5).30 Over a third (12/35) of these indicators measure only commitment or transparency. These include the Marketing category, which depends on companies to voluntarily disclose violations of their internal marketing policies. TTI counts these reports as positive signs of the company’s “transparency”.230 Yet tobacco companies often fail to report marketing violations, as in the case of social media promotion targeted at youth and young adults first uncovered by The New York Times in 2018.6869 The FSFW’s own stakeholder consultation recognised that “youth uptake must be prevented and that this should be explicitly addressed by the Index”;21 yet no indicator in the final ranking addresses the behaviour of tobacco companies towards youth and young adults.
In the “Limitations” section of its final report, TTI also recognises that the reasoning for including the “Capital Allocation” category is flawed. By its own admission, the reason for decreasing the weighting (from 29% to 25%) of this category from preliminary to finalised methodology was: “justified by the consideration that the direct size of financial investment is not necessarily correlated with a positive harm reduction approach”.2
Diverts focus from reducing sales of high-risk products
According to the final TTI methodology weighting of TTI categories (“metrics”) and sub-categories (“indicators”) is reportedly based on the stakeholder consultation and an “analytical hierarchical process”, or “AHP”.30 AHP establishes the relative importance of one element to another. However, this method requires first establishing what makes something more important than something else. In the case of TTI, one of the principles used to do this is to emphasise the importance of increasing relative sales of “reduced-risk” alternatives, “which are weighted higher than indicators solely focused on high-risk products”.30 Within the Product Sales category, arguably the only category included in TTI that measures actual company performance, each subcategory is further divided into “Volume Sales of High-Risk Products” (indicators 2A.1-2) and “Ratio of Volume Sales (Reduced-risk vs High Risk Products)” (indicators 2A.3-4). Indicators 2A.1-2, which in TTI’s own words, represent “a decreasing contribution to ongoing tobacco-related harm, are nonetheless weighted at a mere 5%, whereas Indicators 2A.3-4 are weighted at 45% despite representing only “potential progress in transitioning consumers away from high-risk products”.30 The problem with this approach is that it ignores the capacity of transnational tobacco companies to continue to sell high volumes of combustibles and diverts focus from the essential and evidence-based focus of tobacco control on reducing sales of high risk products.
Opaque calculation of HMIC vs LMIC differential
From the preliminary to final methodology, TTI changed its accounting of differences in tobacco company policy and performance between high- and middle-income countries (HMIC) and low- and middle-income (LMIC) countries from separate indicators to a “LMIC vs HMIC multiplier”.30 Though this multiplier is referenced multiple times in the text of the methods report (e.g. ““Details on the LMIC vs HMIC multiplier are provided in the Approach to Scoring section”30), no such explanation is actually provided. This is especially concerning considering the multiplier is used to calculate companies’ scores for 11 of the 35 total indicators.
Does not account for dual/poly use
The harm reduction framework employed by TTI also does not consider the possibility of dual- and poly-usage of combustible and non-combustible tobacco products. Dual use does not necessarily reduce harm; users are still exposed to high levels of risk from use of high-risk tobacco products. Although products have not been on the market in many countries for long enough to draw conclusions on the general prevalence of dual and poly use, there is a growing body of academic research emerging from Japan (the largest market for PMI’s IQOS),7071 and South Korea,7273 which has found significant levels of dual use of heated tobacco products (HTPs) and conventional cigarettes. Research published in 2020 from South Korea suggest that up to three quarters of youth HTP users who are current cigarette users and are less likely to quit than e-cigarette users.73
There is also some emerging evidence that newer products like heated tobacco devices may be attractive to those who have never smoked cigarettes, including young people.73747576
By failing to put in place sufficient guards against marketing to youth and young adults, the tobacco industry enables youth uptake of newer products and continuing dual- and poly-use.77 TTI does not acknowledge this tobacco industry strategy.
Ignores tobacco industry interference
None of the TTI metrics attempt to capture the ways in which the tobacco industry interferes with policymaking and opposes effective tobacco control measures. The only category that mentions policymaking, Lobbying & Advocacy, contains indicators that solely assess companies’ “disclosure of policy positions related to industry transformation” (6A.1) and disclosure of lobbying and advocacy activities (6A.2).30 In the final report, only three of the report’s 121 pages directly address the tobacco industry’s role in lobbying, and even then, only whether companies disclose their policy positions and lobbying organisation memberships.2 Indicator 6A.1 allows tobacco companies to “score points “ for disclosing policy positions related to tobacco harm reduction;2 what this fails to account for is how these positions are used to combat tobacco control policy worldwide. See, for example, our page on PMI Promotion of IQOS Using FDA MRTP Order.
Though the TTI also originally promised to “document FCTC violations” in the Request for Proposals,9 no further mention is made to this in subsequent documents. In its review, the Expert Panel recognised that FSFW should improve its analysis of the “actual behavior of ranked companies, including at a country level”.25
Tobacco companies actively oppose strong tobacco control measures that would benefit public health.29 More examples of how the industry attempts to interfere with governments across the world to weaken tobacco control can be found in The Global Tobacco Industry Interference Index, produced by GGTC, a partner in STOP.
Relevant Links
- Foundation for a Smoke-Free World website: org/advancing-industry-transformation/
- Tobacco Transformation Index website: tobaccotransformationindex.org
TobaccoTactics Resources
- Foundation for a Smoke-Free World
- Foundation for a Smoke-Free World Archives
- Philip Morris International
- Euromonitor International
- Greenwashing
- CSR Strategy
- CSR: Awards
TCRG Research
- Foundation for a Smoke-Free World is not complementary to public health efforts – it’s undermining them, H. Lusardi, A. Gilmore, U. Dorotheo, Tobacco Control, 4 March 2020
- The Philip Morris-funded Foundation for a Smoke-Free World: tax return sheds light on funding activities, T. Legg, S. Peeters, P. Chamberlain, A.B. Gilmore, The Lancet, 6 June 2019
- Euromonitor International now accepts tobacco industry funding: a win for PMI at the expense of research on the tobacco industry, A.W.A. Gallagher, A.B. Gilmore, Tobacco Control, 8 April 2019
- Understanding the emergence of the tobacco industry’s use of the term tobacco harm reduction in order to inform public health policy, S. Peeters, A.B. Gilmore, Tobacco Control, 2015;24:182-189
- Transnational Tobacco Company Interests in Smokeless Tobacco in Europe: Analysis of Internal Industry Documents and Contemporary Industry Materials, S. Peeters, A.B. Gilmore, PLOS Medicine, 2013;10(9):e1001506
- Latest Tax Return Sheds Light on Philip Morris-funded Foundation for a Smoke-free World, STOP Industry Activity Brief, May 2019
- 10 Takeaways from The Foundation for a Smoke-Free World’s 2019 Tax Return, STOP Industry Brief, May 2020
- Addiction at Any Cost: Philip Morris International Uncovered, STOP report, February 2020